Spain
Country
Hemgenix has now secured reimbursement in several European markets, and while the path to reimbursement for the high-cost product has not always been easy, innovative access deals have helped to smooth the way.
Consumer health manufacturer Uriach has agreed to acquire Germany's Pascoe Naturmedizin and through a separate transaction France's Laboratoires Ineldéa, following investment by private-equity firm ICG.
J&J says it has “exhausted all current viable avenues” to get its antidepressant nasal spray Spravato reimbursed on England’s National Health Service, after NICE decided against re-appraising the drug following numerous funding rejections.
Recent consumer health appointments in Europe and Asia: Italian and Spanish OTC associations elect presidents and vice-presidents; P&G India names Hygiene & Heath Care CFO.
Hikma has announced the establishment of a Spanish subsidiary, marking the firm’s latest push into a new market alongside recent expansions in France and Canada. The moves are in line with a strategy laid out to Generics Bulletin several years ago by current CEO Riad Mishlawi.
Santhera Pharmaceuticals did not provide enough evidence to demonstrate that its Duchenne muscular dystrophy drug Agamree was a cost-effective use of resources, according to draft guidance from England’s health technology assessment body, NICE.
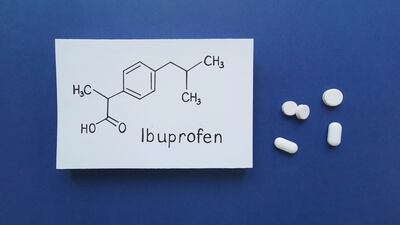
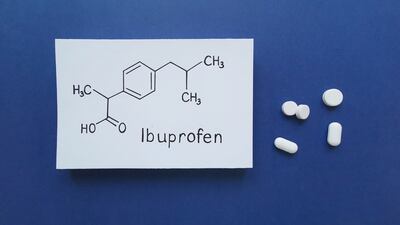
Ibuprofen generic sold by Spain's Liconsa should have its marketing authorization suspended in multiple EU member states as it is not bioequivalent to reference product Nurofen Rapid, rules the European Medicines Agency.
Ibuprofen generic sold by Spain's Liconsa should have its marketing authorization suspended in multiple EU member states as it is not bioequivalent to reference product Nurofen Rapid, rules the European Medicines Agency.
As a number of major brands face generic and biosimilar competition, the loss-of-exclusivity impact across the leading 10 developed markets will approach $200bn over the next five years, according to a fresh medicines spending report running to 2028.
A round-up of the latest appointments in Europe's consumer health market: Cooper Consumer Health names Spain & Portugal head; Orkla Health adds to board; TheOTCLab appoints first Germany manager; Dr. Pfleger hires digital specialist.
A round-up of the latest appointments in Europe's and the US health and wellness industries: Spain's ANEFP elects Martín as president; US CHPA appoints Parks as senior VP of regulatory and scientific affairs, Norgine hires HRA's Hilton to lead its consumer business; Infectopharm names Weleda's Ammendola as R&D head; and DSM-Firmenich makes management changes.
Unveiling plans to install a new bioreactor at its Genhelix site in Spain, mAbxience is bolstering its biosimilars manufacturing capabilities and CDMO capacity under the majority ownership of Fresenius Kabi.
Spain’s Laboratorios Farmacéuticos Rovi has a firm date to remedy issues observed in its application for a hybrid version of risperidone, developed using Rovi’s proprietary ISM drug-release technology platform.
Zentiva has added the Zerinol and Schoum Solution OTC brands to its Italian portfolio. Meanwhile, the firm has also agreed a “major product acquisition” with Tillomed Spain in the hospital oncology segment.
Instagram is now the most important social network for Spanish OTC companies, according to the results of a recent survey by the country’s self-care industry association, ANEFP. Meanwhile, ANEFP predicts 11% growth in the country's self-care market by the end of 2022.
The latest notified body listing brings total number of EU designated Medical Device Regulation testing and certification organizations to 35.
Marine algae phytoplankton is abundant with numerous vitamins and minerals and is found on the ocean’s surface. Blugenics Innovations markets Karen brand powder and tablet line with proprietary phytoplankton ingredient it also provides to other firms.
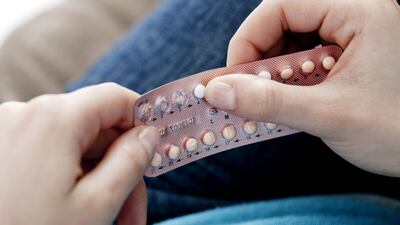
Representatives of doctors and pharmacists have met with the director of the Spanish Agency for Medicines and Medical Devices to discuss a position paper which argues enabling OTC access to the daily contraceptive pill will empower women to manage their own reproductive health and improve family planning.
A study backed by HRA Pharma found that 65% of women in Italy, 55% in Spain and 49% in Germany feel positive about accessing a progestin-only oral contraceptive pill without a prescription. HRA told HBW Insight that the research "confirms it is now time for better access to more effective contraception:"
Spanish competition regulator the CNMC has fined Merck Sharp and Dohme €39m for abusing its dominant position, by blocking Insud Pharma’s rival to the Nuvaring vaginal contraceptive ring through “unjustified legal action” that sought to delay market entry of Insud’s Ornibel version. The originator is considering an appeal.
ADVERTISEMENT