Policy
With the US BIOSECURE Act waiting for a Senate vote, there are signs it may be prompting some Chinese firms to look at their operations. In the meantime, two legal experts in China suggest a range of coping strategies for companies that may be deemed "of concern."
With the US BIOSECURE Act waiting for a Senate vote, there are signs it may be prompting some Chinese firms to look at their operations. In the meantime, two legal experts in China suggest a range of coping strategies for companies that may be deemed "of concern."
Avoiding disaster at the beginning of the pandemic and offering a pro-active supply chain blueprint should position the generics industry well for an onshoring debate in Congress, but a look back at March 2020 is a reminder that even small policy shifts can have a big impact.
Green Pharmaceuticals’ SnoreStop Nasal Spray, previously marketed as “NasoSpray,” still is available even though agency officials on multiple occasions for a month recommended a recall after an April inspection found “gross microbial contamination” in one lot.
After US President Joe Biden launched a review of the country’s API supply chain, the task force has reported back with key vulnerabilities that contribute to drug shortages and supply risks during a global public health emergency. According to the report, the lack of geographic diversity and dependence on foreign nations and anti-competitive actions by foreign nations are key areas of concern.
A fresh executive order from US president Joe Biden calls for short-term and long-term study, broad consultation, and co-ordination with allies on the domestic supply chain.
The generics firm essentially paid the brand to keep its product off the market, FTC alleges in case about Opana ER litigation. Endo considered bringing back its original formulation of oxymorphone but instead reached agreement with Impax to share profits of its generic, antitrust suit claims.
US off-patent industry association the AAM has welcomed the prospect of working with the incoming Biden-Harris administration, emphasizing that there is “more to be done” to improve generic and biosimilar utilization and uptake.
Industry says it is ready to work with the government on the recommendations from a major review of Australia’s health technology assessment system that covers areas such as discount rate reductions and setting up a separate budgetary allocation to temporarily subsidize access to certain drugs.
The Food and Drug Administration’s final guidance outlines the agency’s thinking for sponsors submitting de novo requests electronically, which they must starting in October 2025.
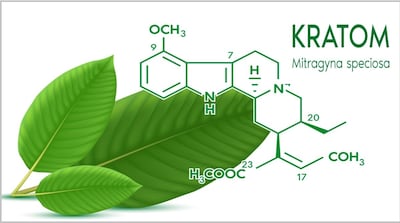
Week after publishing safety alert about OPMS Black Liquid Kratom “linked to serious adverse health effects, including death,” FDA announced market research “to understand and characterize emergent risk/safety and perceived benefits reportedly linked to kratom and psychedelics.” But it withdrew the study 10 days later.
Brazil looks to EU, UK and others for inspiration on introducing a regulatory sandbox.
After 10 years of promised investment following its Nobel Prize for iPS cell research, Japan is cautiously narrowing regulations around the conditional approval of cell therapies and cutting some reimbursement prices. Commercial success remains mixed and some products have been withdrawn from the market.
After 10 years of promised investment following its Nobel Prize for iPS cell research, Japan is cautiously narrowing regulations around the conditional approval of cell therapies and cutting reimbursement prices. How has the sector panned out over the last decade?
Semi-annual regulatory agenda of non-binding target dates also sets December goal for an NPRM to recognize N-acetyl-L-cysteine as a lawful dietary ingredient. Like IND exemptions rule, item on NAC included for first time in FDA’s list.
Neither Kamala Harris nor Donald Trump mentioned drug pricing reforms in their presidential convention speeches.
This HBW Insight series profiles regulatory affairs specialists working in or supporting the consumer health and beauty product industries. In this installment, we speak to Kenvue's Kevin Whelan.
Multi-cancer diagnostics can help get oncology patients the treatment they need more quickly, but lack of reimbursement has kept such tests out of reach for many patients. Bills providing coverage have passed or are under consideration in more than half of the states and have been introduced in both houses of US Congress.
Medicare coverage with evidence development final guidance includes few substantive changes from the proposed version despite biopharma stakeholder concerns.
ADVERTISEMENT