Pediatrics
Cardiovascular and Renal Drugs Advisory Committee will consider whether open-label extension data from a randomized trial that failed its primary endpoint, along with a historical control comparison, are enough to support approval in the ultra-rare disease.
Under current law, drugs or biologics must receive rare pediatric disease designation before 1 October to be eligible for a priority review voucher. The sunset provisions have led to a spike in designation requests at the FDA and a push by pediatric and rare disease advocates for an extension, either through a continuing resolution or reauthorizing legislation.
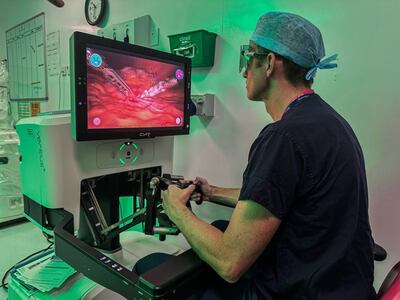
CMR Surgical has launched a multicenter pediatric clinical trial for its Versius surgical robot system across three UK sites. The trial will enroll 150 children for urological procedures, with patient outcomes followed for up to a year post-surgery.
Warning letters to SuXiang Medical Instrument in China and Yahon Enterprise in Vietnam among recent warnings FDAS sent to OTC drug and supplement manufacturers, including a Florida firm, White Label Leaf, warned about selling gummies containing delta-8 THC, and other OTC skin care product firms.
As the reauthorization deadline nears, CDER and CBER leaders stressed that sunsetting the program would hurt pediatric rare disease development, while endorsing a new Democrat-proposed stick for rare disease research.
Senior leadership in the FDA’s drugs, biologics and devices centers want reviewers to feel more comfortable taking risks in product approvals, but may need more concrete examples of regulatory flexibility's success to convince them.
With the axe falling on a growing number of essential legacy products, Tom Melvin explains why a plan around derogations for devices falling through the regulatory crack is now critical.
Senior leadership in the FDA’s drugs, biologics and devices centers want reviewers to feel more comfortable taking risks in product approvals, but may need more concrete examples of regulatory flexibility's success to convince them.
Kenvue plans to ask judge to dismiss complaints relying on testimony from latest expert plaintiffs’ attorneys offered that prenatal use of Tylenol or other acetaminophen oral products is linked autism and ADHD in children. Judge previously dismissed complaints relying on five other experts’ testimony.
Three new vaccines to prevent RSV infections in seniors and sales trends in pediatric vaccines highlight a shift away from childhood vaccines and a possible age-related immunity deficit.
A recent review of orthopedic device approvals between 2018 and 2022 found that less than 10% of 510(k) devices – and less than 5% of PMA and de novo products – have been authorized for use in children, highlighting the ongoing lack of pediatric devices.
Perrigo’s adjusted operating income from formula business in 2023 was around half the $140m it expects. Level won’t be reached in 2024 but it’s “well on track to recapturing” that amount in 2025 and to “capturing all of that plus in 2026,” says CEO Patrick Lockwood-Taylor.
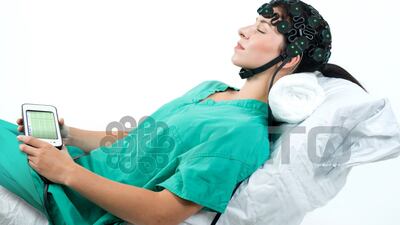
The US FDA has granted 510(k) clearance to a California medical technology company for a brain monitoring device that detects seizures in patients with brain injury or trauma.
The push for more transparency so researchers are not unnecessarily duplicating or competing for the same small pool of patients raises a problem that goes beyond the RACE Act, FDA officials said.
Michael Creaturo’s petition isn’t the first suggestion made to FDA and industry that there’s room for improvement in labeling for children’s doses of some OTC formulations. Creaturo's petition comes after he received a US patent in December 2022 for an “integrated container and dosing device for liquid medication delivery.”
Initial data looks promising as the FDA’s pediatric oncology subcommittee is set to discuss how recent legislative changes have impacted pediatric cancer drug development.
Plezi also announces launches “PLEZi FiZZ” carbonated fruit drink with 70% less sugar than the same amount of leading soft drinks, no added sugar, plus 2g fiber, vitamin C and other nutrients. With 10% of profits going to children’s health initiatives, its initial donation was $1m to FoodCorps' Nourishing Futures.
Nicole Verdun said children could participate in gene therapy clinical trials earlier if the necessary controls are in place.
Great Ormond Street Hospital is piloting a new approach to gene therapy delivery in which it will submit a marketing authorization application to the UK regulator for a lentiviral gene therapy used to treat ADA-SCID, a rare disease.
Nutritional product business had 5.1% Q1 sales growth and is like Abbott’s other segments, “super well-aligned to the global demographics and trends in health care,” says CEO Ford. But as it defends complaints of damages from powder formulas made at facility found with unsafe levels of bacterial contaminants, Abbott’s also targeted in litigation alleging failure to warn about risk of infants born prematurely developing necrotizing enterocolitis if fed cow’s milk-based formula.
ADVERTISEMENT