International
Green Pharmaceuticals’ SnoreStop Nasal Spray, previously marketed as “NasoSpray,” still is available even though agency officials on multiple occasions for a month recommended a recall after an April inspection found “gross microbial contamination” in one lot.
The Medicines Patent Pool has entered into a partnership with three companies for glecaprevir/pibrentasvir, a WHO-recommended treatment for people living with hepatitis C. The companies join previous generic licensee Mylan and originator AbbVie.
Pfenex founder and chief business officer Patrick Lucy has left the company to join Lykan Bioscience. Meanwhile, Samsung Biologics has announced the appointment of a new president and CEO, as the Australian generics and biosimilars association appoints a new chair.
Stay up to date on regulatory guidelines from around the world with the Pink Sheet's Guidance Tracker. The complete Global Pharma Guidance Tracker, with sortable and searchable listings going back to 2014, is available online.
Stay current on regulatory guidelines from around the world with Medtech Insight's Guidance Tracker. Over 40 documents have been posted on the tracker since its last update.
Stay up to date on regulatory guidelines from around the world with the Pink Sheet's Guidance Tracker. The complete Global Pharma Guidance Tracker, with sortable and searchable listings going back to 2014, is available online.
Stay current on regulatory guidelines from around the world with Medtech Insight's Guidance Tracker. Nearly 80 documents have been posted on the tracker since its last update.
With concern over the mpox health emergency continuing to build, the Medicines Patent Pool has offered its services to play a part in the global response.
Marking another step in Dr Reddy’s diverse corporate strategy, the company has signed a memorandum of understanding with Kainomyx for the development and commercialization of anti-malarials in multiple markets.
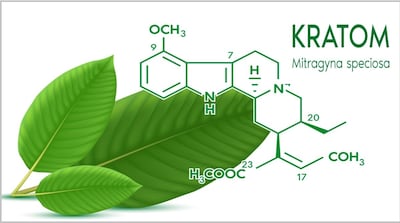
Week after publishing safety alert about OPMS Black Liquid Kratom “linked to serious adverse health effects, including death,” FDA announced market research “to understand and characterize emergent risk/safety and perceived benefits reportedly linked to kratom and psychedelics.” But it withdrew the study 10 days later.
Semi-annual regulatory agenda of non-binding target dates also sets December goal for an NPRM to recognize N-acetyl-L-cysteine as a lawful dietary ingredient. Like IND exemptions rule, item on NAC included for first time in FDA’s list.
This HBW Insight series profiles regulatory affairs specialists working in or supporting the consumer health and beauty product industries. In this installment, we speak to Kenvue's Kevin Whelan.
To help sponsors developing candidate mpox vaccines, the World Health Organization will soon be finalizing guidance on the preferred and minimum characteristics these products must satisfy regarding efficacy, dose regimen and other aspects to secure regulatory approval.
Driven by increased orders, Alvotech is bullish on its US adalimumab biosimilar moving into 2025, as it also look forward to the US launch for its Stelara biosimilar early next year.
Generics Bulletin reviews the latest regulatory events across the world.
Conflicted experts should be allowed to participate as nonvoting members and panels should take a benefit-risk vote on product-specific applications, the majority of respondents said in a survey conducted by 3D Communications.
Viome Life Sciences launches VRx MyBiotics Toothpaste & Gel to complete its Oral Health Solution system; Liquid Core Gum’s Edge Performance made with its Liquid Core and Flavor Wave properties and distributed in packaging developed using sustainable cardboard tubes; and NOW Health Group launches Omega-3 Gummy Chews and E-Sport Reaction supplements.
Plastics – specifically the reduction of virgin plastic in packaging and the move to recyclable or refillable packaging – is one of nine priority topics Kenvue is focused on as part of its global sustainability strategy, according to the company’s global head of packaging innovation, sustainability and experience, David Lickstein.
Height of plaintiff attorney’s argument to present evidence which would prompt speculation by a jury was request to parse research by Kenvue’s lead expert, who coordinated the International Consensus Statement on ADHD by the World Federation of ADHD, where he’s president.
A round-up of the latest global health and wellness moves: Nestlé and Dr Reddy's name JV head; Be-sup hires comms and regulatory director; Ascendis Health appoints interim CFO.
ADVERTISEMENT