Enforcement
Green Pharmaceuticals’ SnoreStop Nasal Spray, previously marketed as “NasoSpray,” still is available even though agency officials on multiple occasions for a month recommended a recall after an April inspection found “gross microbial contamination” in one lot.
The generics firm essentially paid the brand to keep its product off the market, FTC alleges in case about Opana ER litigation. Endo considered bringing back its original formulation of oxymorphone but instead reached agreement with Impax to share profits of its generic, antitrust suit claims.
This week, two device testing labs in China landed FDA warning letters; refunds for 1Health.io clients; FDA AR/VR product list expands.
AbbVie’s migraine ad overstates the drug’s benefit, a problem that is amplified by using a celebrity, the agency says.
This week, a Delaware court awarded Auris Health shareholders $1bn in a lawsuit against Johnson & Johnson; Abbott recalled some FreeStyle Libre 3 sensors; and McKesson purchased a controlling interest in a Florida cancer care chain.
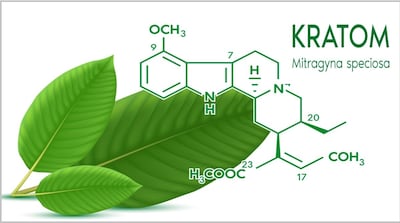
Week after publishing safety alert about OPMS Black Liquid Kratom “linked to serious adverse health effects, including death,” FDA announced market research “to understand and characterize emergent risk/safety and perceived benefits reportedly linked to kratom and psychedelics.” But it withdrew the study 10 days later.
Dietary supplements containing unauthorized novel foods were reported to the European Commission by national regulators on around 40 occasions in the second quarter of 2024.
This week, surgical robot maker Globus Medical got a warning letter from the US Food and Drug Administration; the FDA cleared a hemostatic gel to stop blood loss; Medicare issued a payment code for Medtronic’s renal denervation device; and more.
Request to recall eye drops should be fulfilled promptly and businesses providing lip balms as promotional products must verify contract manufacturers are compliant, recent FDA warnings states. Additional letters went to Jordanian firm about testing alcohol for methane and to a Chinese firm advised that compliance with China’s quality control standards isn’t sufficient.
The US government continued its focus on False Claims Act enforcement and collected funds totaling more than $1bn in the first of this year alone – $155m of which was medtech-related.
The lung cancer drug Krazati from Bristol Myers Squibb’s Mirati Therapeutics drew an FDA ad/promo untitled letter, highlighting the agency's trend of pursuing false or misleading efficacy claims, particularly when data comes from outside approved labeling.
As it has every year since FSMA was passed in 2011, FDA doesn’t plan to impose reinspection fees until it publishes guidance for small businesses to request reductions. FY2025 budget proposal includes plan “to re-structure the fee programs to make it more administratively feasible to operate.”
The US Food and Drug Administration released five warning letters and two close-outs last month, including two warning letters to Chinese makers of plastic syringes.
CRN appreciates Durbin’s “commitment to greater transparency in the dietary supplement marketplace,” but “cannot endorse it in its current form”; AHPA asks for better option; and NPA remains opposed.
Warning letters to SuXiang Medical Instrument in China and Yahon Enterprise in Vietnam among recent warnings FDAS sent to OTC drug and supplement manufacturers, including a Florida firm, White Label Leaf, warned about selling gummies containing delta-8 THC, and other OTC skin care product firms.
This week, Edwards announced that it has purchased JenaValve and Endotronix; a New Jersey lab has agreed to pay the government $5m for violating anti-kickback law; eCential Robotics’ spine platform made its debut for human use; and more.
The UK’s drug regulator said that a trial run by cell therapy firm Celixir “risked seriously jeopardizing the rights, safety and wellbeing of trial participants” after an inspection discovered expired product batches, unauthorized dosing and other serious breaches.
A New York district court ordered the asset freeze after finding that the owners of H&H Wholesale Services had concealed or gambled away money owed to Abbott. The company was ordered to pay Abbott $33m in 2023 for distributing test strips authorized for sale only outside the US.
Attorneys discuss potential impacts on consumer health products industry from Supreme Court’s “Loper Bright” decision in June on litigation brought by two fisheries, Loper Bright v. Raimondo and Relentless v. Department of Commerce.
Noting similar warnings a year ago, the FDA and FTC announce warnings to six more, part of a joint effort to stop sales of copycat food products containing delta-8 THC, saying companies selling these "illegal products are demonstrating complete neglect for consumer safety.”
ADVERTISEMENT