E-Commerce
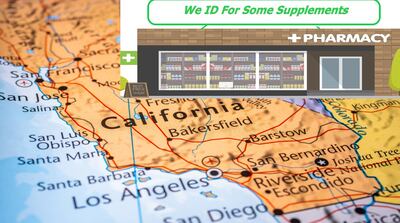
California Senate Appropriations Committee suspends consideration of bill for current session after it and Judiciary Committee voted to recommend passing the bill earlier in session. Legislative sessions continue in Massachusetts and New Jersey with bills for similar restrictions.
The FTC says its final rule banning fake consumer reviews and testimonials will help to restore its depleted toolbox for obtaining civil penalties. The agency maintains the final rule’s benefits will greatly outweigh costs for honest companies increasingly set back by bad actors in the space.
WishGarden Herbs marks 45 years with line extensions; Obesity doctor develops SoWell supplements for GLP-1 patients; Vital Proteins makes sustainability vital in packaging; and interior decoration and rent awards in vitaminwater campaign.
Herbalife adds distributors and training as sales slow; Amphastar bullish on Primatene Mist $100M full-year target; and ChromaDex net sales up 12%.
Kenvue maintains full-year guidance after 0.3% Q2 net sales dip beat market expectations. Essential health segment alone drove sales; self-care and skin health/beauty faltered. US sales of Neutrogena Collagen Bank pre-aging platform launched on TikTok shop, a first for the brand.
Church & Dwight’s slower sales at retail in June-July than in January-May point to still slower results for rest of year. It doesn’t expect second-half help from gummy vitamin lines or give them a full vote of confidence for remaining in portfolio.
L’Oréal's European business recorded sales growth of 9.7% to €3.55bn in the second quarter as consumer confidence grows, while in the US sales slowed for the dermatological beauty business and consumers in China are value-shopping.
P&G's FY2024 Q4 net sales were flat at $20.5bn, lower than consensus estimates, but the firm says its underlying business divisions are healthy and forecasts FY2025 sales up 2% to 4%, up 3% to 5% organically.
CRN appreciates Durbin’s “commitment to greater transparency in the dietary supplement marketplace,” but “cannot endorse it in its current form”; AHPA asks for better option; and NPA remains opposed.
Warning letters to SuXiang Medical Instrument in China and Yahon Enterprise in Vietnam among recent warnings FDAS sent to OTC drug and supplement manufacturers, including a Florida firm, White Label Leaf, warned about selling gummies containing delta-8 THC, and other OTC skin care product firms.
Martha Stewart sells supplements on iHerb; Cheribundi partners spread the brand at Paris Games; GoodSport’s latest partner is a Bear; Herbalife-sponsored runner competes in ultramarathons; Dutch researcher joins Unicity science board; and Beacon Wellness adds sales strategy chief.
Analysts tracking consumer health product firms in the US anticipatevApril-June results lower than a year ago across most businesses. While some results will be lower on comparisons with strong growth in 2023, others will reflect a slow allergy season, retailers’ inventories remaining high as consumer spending slows or firms continuing to recover from supply chain problems.
Bumble and bumble follows Too Faced and Clinique, also with their own storefronts within Amazon’s Premium Beauty space. Lauder notes the broader reach and the importance of meeting consumers where they already are shopping.
Industry can look forward to a global OTC market compound annual growth rate of around 7% over the next few years, according to IQVIA Consumer Health. Innovation will be key to ensure individual companies realize this level of growth. IQVIA CH's Amit Shukla and Simon-Kucher & Partners' Clemens Oberhammer point to some key consumer trends to keep an eye on at the recent AESGP Annual Meeting in Brussels.
During recent investor conference, president Stephan Gratziani and CFO John DeSimone discuss Herbalife’s restructuring in addition to progress on digital transformation program and lack of progress on expectations for marketing to consumers prescribed GLP-1 drugs for weight loss.
International Food Additives Council suggests master file system will be redundant to submitting NDINs required for some dietary ingredients. IFAC also asks FDA to clarify whether the history of a substance’s use in food “is at the strain level or species level.”
CRN is “disappointed the draft guidance was not accompanied with an announcement by FDA of its commitment to rigorously enforce the NDI notification requirement.” CHPA says NDIN “requirement is only meaningful if FDA consistently takes enforcement action against manufacturers and distributors” noncompliant with the requirement.
Latest moves on selling to consumers using GLP-1 receptor agonist drugs for obesity come from firms as large as global food products manufacturer and marketer Nestle and as small as startup Promino and as ambitious as men’s health product marketer Ro offering an app at no charge to find GLP-1 drugs.
Senate committee amends bill to lower to $250 the largest civil penalty for violations; exempt retail clerks from “disciplinary action or discharge by the retail establishment” related to violations; and make changes to state’s Health and Safety Code effective in January 2026.
Where a US hemp product firm stands on whether de-scheduling the botanical as a controlled substance in the 2018 farm bill left a loophole allowing chemically derived and potentially intoxicating cannabinoid strains to qualify could determine whether it agrees with the detour.
ADVERTISEMENT