Diagnostics
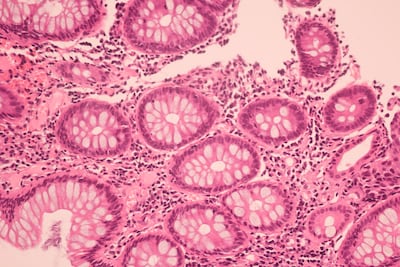
Biopharmaceutical giant AstraZeneca has partnered with start-up “unicorn” Owkin to develop an AI-powered tool to prescreen for gBRCA mutations on the basis of morphological features in digitized pathology slides. Built on extensive, high-quality data sourced from the France-based PortrAIt consortium, the AI will help to prioritize patients for further testing, streamlining the diagnostic process, Owkin says.
This week, Neuralink announced it received US FDA breakthrough device designation for a device to restore sight; medtechs Discure and DeepLook secured new funding; FDA pump recalls from B. Braun Medical and Fresenius Kabi; Axonics prevails in patent infringement lawsuit with Medtronic; Merit Medical buys Cook Medical for $210m.
Medtech Insight interviewed Meri Beckwith, co-founder of “anti-CRO” clinical research organization Lindus Health, about his company’s efforts to make clinical trials more efficient and reliable.
The New York-based augmented intelligence health care services provider has secured $20.7m in series B funding to support clinical validation and commercialization of its AI-powered breast cancer morphology diagnostic test PreciseBreast. The company intends for its test to advance current approaches to recurrence prediction comprising clinician histopathology assessment and genomic testing.
This week, a medical group sued the FDA to block a lab-developed test rule; the FDA published guidance on device classifications; Defibtec issued a recall of its chest compression device and ICU Medical updated instructions for its infusion pump batteries; Maui Imaging raised a $4m DOD grant to put imaging tech into military-based trauma units.
Multi-cancer diagnostics can help get oncology patients the treatment they need more quickly, but lack of reimbursement has kept such tests out of reach for many patients. Bills providing coverage have passed or are under consideration in more than half of the states and have been introduced in both houses of US Congress.
The US FDA has published draft guidance for predetermined change control plans for medical devices along with recommendations for sponsors including them in marketing submissions to the agency.
William Audeh, chief medical officer at Agendia, is optimistic about study results suggesting the company’s genomic test MammaPrint, designed to predict which women with early-stage HR+ breast cancer are at risk of recurrence, also can be used to determine those that would benefit from extended endocrine therapy.
Meron Mathias is vice president of CSR and sustainability at Thermo Fisher, whose specialty diagnostics division makes it the fifth leading player by revenues in the global IVD industry. In this podcast, she reflects on Thermo Fisher’s early commitment to ESG and sustainability reporting, its Scope 1, 2 and 3 targets and how regulation is moving industry towards a mandatory disclosure landscape.
Cresilon CEO Joe Landolina says the newly FDA-cleared Traumagel for moderate and severe bleeding is easier to use than many currently available solutions like gauzes and sponges, and provides faster results.
The US FDA has granted marketing authorization to NOWDiagnostics for the first at-home over-the-counter test to detect syphilis, which the government says is on the rise.
The FDA designated class II special controls classifications for two diagnostics and force separation catheters.
This week, Medtronic recalled a nerve monitoring system due to reports of false responses. The US FDA approved the first auto-injector for opioid-overdose, made by Purdue Pharma. The agency granted de novo authorization for Labcorp’s PGDx elio plasma focus Dx used by labs for genetic profiling. As of 7 August, 950 AI/ML devices have been approved by the FDA. EKO Health teamed up with LSU to help detect arrhythmias and murmurs in student-athletes.
Unilabs and C2N Diagnostics signed a multi-year agreement that will expand access to C2N’s Alzheimer’s tests in Europe and other countries.
In an interview with Medtech Insight, Matt Bettonville, investor at cancer-focused venture firm Yosemite, discussed its criteria for evaluating potential investments in oncology and his outlook on the future oncology landscape.
In this episode of the In Vivo podcast, Billy Boyle, CEO of Owlstone Medical, discusses how his company is developing several breath biopsy products for use by clinicians, biopharma companies and researchers.
This week, the US FDA sent a warning letter to maker of batteries for AEDs, AMCO; Virtual Incision successfully completed the first hysterectomy its miniaturized robotic-assisted surgery device MIRA; The DOJ finalized a rule that requires government-operated health care facilities to provide accessible equipment for people with disabilities; the FDA compiled its resources on reprocessed medical devices onto a new web page; and more.
GE HealthCare announced it will team up with AWS to build new generative AI models and applications that can help doctors find key patient information faster and help with diagnosing and treating patients.
On 29 July, Guardant Health Inc. announced it received US FDA approval for its blood test Shield to be used as a primary screening option for colorectal cancer, bringing it one step closer to gaining Medicare coverage.
In June, the US Supreme Court reversed the Chevron doctrine, a long-standing precedent requiring courts to defer to regulatory agencies when statutory language was ambiguous. But will that decision prevent the FDA’s final rule on laboratory developed tests from taking effect? A legal expert weighs in.
ADVERTISEMENT