Consumer
Green Pharmaceuticals’ SnoreStop Nasal Spray, previously marketed as “NasoSpray,” still is available even though agency officials on multiple occasions for a month recommended a recall after an April inspection found “gross microbial contamination” in one lot.
PATIENT ACCESS TO UNAPPROVED MEDICAL PRODUCTS WOULD BE ALLOWED under legislation introduced by Rep. Peter DeFazio (D-Ore.) on July 12 and Sen. Tom Daschle (D-S.D.) on July 14. Called the Access to Medical Treatment Act, the legislation would allow health practitioners to administer unapproved drugs, devices, foods or dietary supplements to patients as long as there is no evidence the treatment causes harm and the patient is informed about the treatment and its possible side effects. DeFazio's bill is identified as HR 2019; Daschle's version will be assigned a bill number the week of July 17.
Dexcom announced the launch of Stelo, the first non-prescription continuous glucose monitoring system to hit the US market, but is likely going to face competition soon from Abbott.
In this episode of the Over the Counter podcast, HBW Insight speaks to Kerry's RDA senior manager of immune and joint health, Sonja Nodland, about the opportunity for innovation that postbiotics represent for the consumer health industry.
Proposal to have panelists rate the strength of efficacy and safety evidence on a numeric scale also draws support in written comments on ways to optimize the advisory committee process.
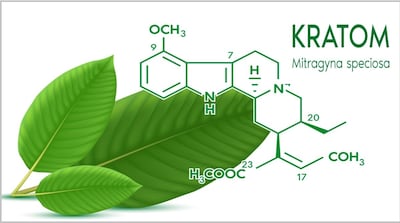
Week after publishing safety alert about OPMS Black Liquid Kratom “linked to serious adverse health effects, including death,” FDA announced market research “to understand and characterize emergent risk/safety and perceived benefits reportedly linked to kratom and psychedelics.” But it withdrew the study 10 days later.
Angelina Jolie For TOM FORD; Haus Labs by Lady Gaga; Gillette Targets Gamers; Cosmetic News In Brief
Estee Lauder Companies’ TOM FORD signs Academy Award-winning actress Angelina Jolie as the face of its Runway Lip Color Campaign launching 3 September. Dolly Parton introduces her first beauty range, Dolly Beauty, through Scent Beauty. More cosmetics and personal-care news in brief.
The New York-based augmented intelligence health care services provider has secured $20.7m in series B funding to support clinical validation and commercialization of its AI-powered breast cancer morphology diagnostic test PreciseBreast. The company intends for its test to advance current approaches to recurrence prediction comprising clinician histopathology assessment and genomic testing.
Semi-annual regulatory agenda of non-binding target dates also sets December goal for an NPRM to recognize N-acetyl-L-cysteine as a lawful dietary ingredient. Like IND exemptions rule, item on NAC included for first time in FDA’s list.
This HBW Insight series profiles regulatory affairs specialists working in or supporting the consumer health and beauty product industries. In this installment, we speak to Kenvue's Kevin Whelan.
Weak demand for virus-blocking nasal sprays since the pandemic has left Austrian biotech Marinomed with mounting debts. The company has applied for restructuring proceedings with an Austrian court in an attempt to secure its future.
CEO Sue Nabi says Coty has created a “multi-functional, stand-alone organization” within its Consumer Beauty business to drive rapid-fire innovation. In a 20 August presentation, Nabi said the firm’s innovation pipeline undergirds its fiscal 2025 outlook of 6%-8% growth.
Conflicted experts should be allowed to participate as nonvoting members and panels should take a benefit-risk vote on product-specific applications, the majority of respondents said in a survey conducted by 3D Communications.
Viome Life Sciences launches VRx MyBiotics Toothpaste & Gel to complete its Oral Health Solution system; Liquid Core Gum’s Edge Performance made with its Liquid Core and Flavor Wave properties and distributed in packaging developed using sustainable cardboard tubes; and NOW Health Group launches Omega-3 Gummy Chews and E-Sport Reaction supplements.
Perrigo aims its new SolpaOne high-strength paracetamol effervescent at UK pharmacists, who now have expanded powers as part of the UK government's Pharmacy First initiative.
OTC erectile dysfunction treatment Eroxon has made it to the Nordics through local distributor Navamedic, which has reported strong consumer uptake.
Plastics – specifically the reduction of virgin plastic in packaging and the move to recyclable or refillable packaging – is one of nine priority topics Kenvue is focused on as part of its global sustainability strategy, according to the company’s global head of packaging innovation, sustainability and experience, David Lickstein.
Height of plaintiff attorney’s argument to present evidence which would prompt speculation by a jury was request to parse research by Kenvue’s lead expert, who coordinated the International Consensus Statement on ADHD by the World Federation of ADHD, where he’s president.
The beauty giant expects organic sales growth for fiscal 2025 to range between -1% and 2%, falling well short of analyst consensus of 7.2%, reflecting the firm’s overexposure in China and Asia travel retail. A new CEO is incoming with Fabrizio Freda set to step down in June 2025.
A round-up of the latest global health and wellness moves: Nestlé and Dr Reddy's name JV head; Be-sup hires comms and regulatory director; Ascendis Health appoints interim CFO.
ADVERTISEMENT