Business Strategies
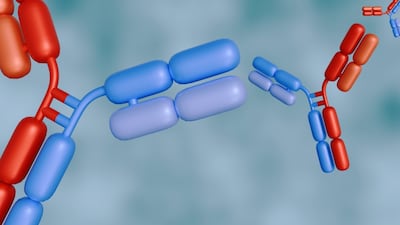
Ordspono, a bispecific antibody newly approved in the EU to treat follicular lymphoma and diffuse large B-cell lymphoma, joins Libtayo in Regeneron’s growing oncology portfolio.
Nemluvio is a first-in-class IL-31 inhibitor that the US FDA approved for prurigo nodularis.
CEO Bill Anderson would not be drawn on whether the drug, currently approved for chronic kidney disease associated with type 2 diabetes, will hit a previous forecast of €3bn peak sales, but expansion into heart failure may make that figure look conservative.
Francesco Hofmann, head of R&D at the mid-sized French group, tells Scrip that its strategy of not playing in the spaces that are dominated by big pharma is paying off.
Johnson & Johnson’s drug for treatment-resistant depression got off to a slow start when it launched in 2019, but strong sales growth has put it on a blockbuster trajectory this year.
This week, Neuralink announced it received US FDA breakthrough device designation for a device to restore sight; medtechs Discure and DeepLook secured new funding; FDA pump recalls from B. Braun Medical and Fresenius Kabi; Axonics prevails in patent infringement lawsuit with Medtronic; Merit Medical buys Cook Medical for $210m.
Edwards Lifesciences’ Critical Care business “invented the hemodynamic monitoring category, and its solutions are currently used in more than 10,000 hospitals globally to better understand the cardiovascular condition in real-time for critically ill patients, which helps improve outcomes,” says BD, which sees synergies and new innovation opportunities across the groups’ data sets and platforms. Acquired for $4.2bn cash, Critical Care generated more than $900m in revenue in 2023.
The Danish firm’s dermatology products have made significant strides in the past six months, leading to an upwards revision of its sales forecast for full-year 2024.
In this episode of the Over the Counter podcast, HBW Insight speaks to Kerry's RDA senior manager of immune and joint health, Sonja Nodland, about the opportunity for innovation that postbiotics represent for the consumer health industry.
Deal Snapshot: The Belgian company follows a tradition of Western pharma companies relying on local expertise to market their products in China.
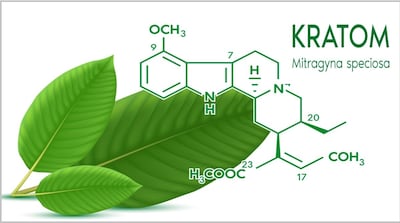
Week after publishing safety alert about OPMS Black Liquid Kratom “linked to serious adverse health effects, including death,” FDA announced market research “to understand and characterize emergent risk/safety and perceived benefits reportedly linked to kratom and psychedelics.” But it withdrew the study 10 days later.
This week, a medical group sued the FDA to block a lab-developed test rule; the FDA published guidance on device classifications; Defibtec issued a recall of its chest compression device and ICU Medical updated instructions for its infusion pump batteries; Maui Imaging raised a $4m DOD grant to put imaging tech into military-based trauma units.
While 2024 has seen two buyouts of antibody-drug conjugate specialists with $1bn-plus price tags, the focus on ADCs has continued, accounting for almost half of recent oncology deals.
Getinge says it plans to pay about $477m for organ transport and services company Paragonix Technologies in an effort to “redefine the market standard in transplantation.”
Plus deals involving Lilly/Oblique, Rafael Holdings/Cyclo, ADCendo/Multitude, Ocuvex/Visiox and more.
This HBW Insight series profiles regulatory affairs specialists working in or supporting the consumer health and beauty product industries. In this installment, we speak to Kenvue's Kevin Whelan.
Weak demand for virus-blocking nasal sprays since the pandemic has left Austrian biotech Marinomed with mounting debts. The company has applied for restructuring proceedings with an Austrian court in an attempt to secure its future.
CEO Sue Nabi says Coty has created a “multi-functional, stand-alone organization” within its Consumer Beauty business to drive rapid-fire innovation. In a 20 August presentation, Nabi said the firm’s innovation pipeline undergirds its fiscal 2025 outlook of 6%-8% growth.
OTC erectile dysfunction treatment Eroxon has made it to the Nordics through local distributor Navamedic, which has reported strong consumer uptake.
ADVERTISEMENT